 FDA regulations have always required careful and comprehensive documentation. The FDA, however, mentioned that there are concerns “centered on whether [the 510(k) program] allows devices to enter the market without sufficient safety and effectiveness evidence,” according to an Aug. 4 FDA news release.
FDA regulations have always required careful and comprehensive documentation. The FDA, however, mentioned that there are concerns “centered on whether [the 510(k) program] allows devices to enter the market without sufficient safety and effectiveness evidence,” according to an Aug. 4 FDA news release.
Meticulous management of design information helps a company record the safety and effectiveness of a product. “To streamline product introduction, many medical device manufacturers are moving away from high-end point solutions for design, analysis, and product data management and toward integrated product development platforms that simultaneously address all of these functions,” said Rich Allen, manager of PDM product management for DS SOLIDWORKS.
“This unified approach accelerates validation of innovative design concepts and automates design documentation, helping companies navigate FDA approvals and stay focused on product development.” Bristol-Myers Squibb Company, DJO Incorporated, Dräger Medical, Kinematic Automation, MAKO Surgical Corp., and Southmedic Inc. are among the companies that use SOLIDWORKS® software products to automate design, analysis, and data management. Design documentation.
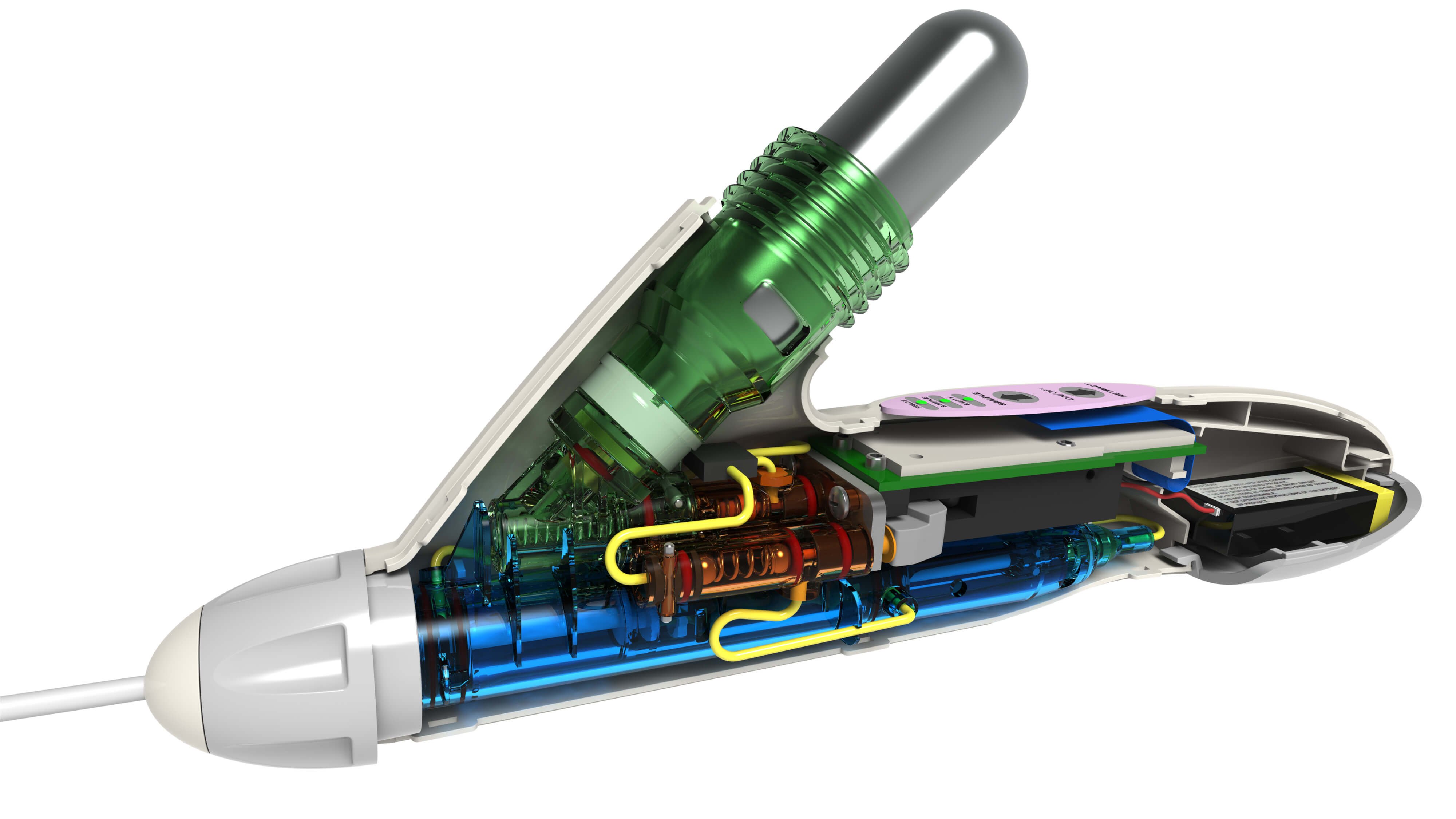
This is the bedrock of FDA approvals. Device makers introducing new products need to be ready to supply regulators with design details, documented design histories, and proof of a device’s safety. Modeling a product in SOLIDWORKS CAD software automatically captures all design data and automates the production of a wide range of documents, including detailed drawings, sections, orthographic views, bills of material, photorealistic renderings, animations, and fly-throughs. Safety and performance analysis.
The FDA expects written finite element analysis results to be submitted with Class II and Class III devices – e.g., those that administer fluids or are implanted. Many medical device makers hire specialized analysts who may use complex software for analysis. Designers and engineers, however, can actually perform many of these tests themselves in SOLIDWORKS Simulation software, which is integrated into the SOLIDWORKS design environment. Data management.
Many device makers lose time and vital information by storing design documentation and safety data separately from their design environment, in manual, paper, or high-end enterprise management systems. With SOLIDWORKS, design, simulation, and data management is a cost-efficient and seamlessly integrated process with full audit trails. Regulatory compliance aside, SOLIDWORKS products can help medical device makers: Develop ergonomically friendly products that may shorten medical procedures, improve outcomes, and appeal to health-care professionals.
Predict behavior of new sophisticated device materials, including strength, conductivity, and ability to be sterilized. Ensure durability and dependability of implants Quickly obtain reference designs, including models of patients’ bones, from 3D scans Create photorealistic images and animations for part and assemblies for marketing and educational purposes Focus on their core competencies and intellectual property, not managing complex information technology. By using powerful design software and a comprehensive design strategy,” Allen said, “companies can quickly and cost-effectively bring new products to market that can make an immediate impact on patients’ lives.”
These imperatives are discussed at length in two SOLIDWORKS white papers “Bringing Innovative Medical Products to Market Faster” and “Analysis guide for Medical Product Designers.”